Learning Outcomes:
i. Define aliphatic and aromatic hydrocarbons.
ii. Identify and differentiate between aliphatic and aromatic hydrocarbons based on their structure.
iii. Recognize the open-chain and branched-chain structures of aliphatic hydrocarbons.
iv. Understand the cyclic and delocalized nature of aromatic hydrocarbons.
v. Appreciate the significance of hydrocarbon classification in organic chemistry.
Introduction
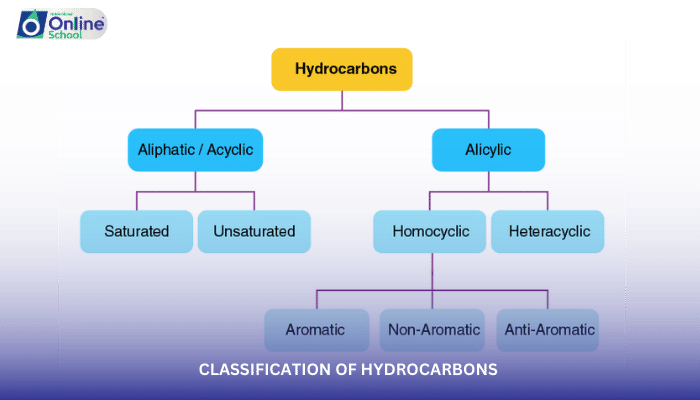
Hydrocarbons, the simplest organic compounds, consist solely of carbon and hydrogen atoms. They form the basis of many other organic compounds and exhibit a wide range of properties, from the gaseous methane (CH4) to the solid polyethylene (C2H4)n. In this introductory lesson, we will explore the classification of hydrocarbons into two major categories: aliphatic and aromatic.
i. Aliphatic Hydrocarbons: Open-Chain and Branched-Chain Structures
Aliphatic hydrocarbons are characterized by their open-chain or branched-chain structures. They lack the ring structure found in aromatic hydrocarbons. Aliphatic hydrocarbons can be further classified based on the type of carbon-carbon bonds they contain:
Alkanes: Alkanes contain only single bonds between carbon atoms (C-C) and have a general formula of CnH2n+2. They are generally unreactive and form the basis of many natural fuels, such as natural gas and propane.
Alkenes: Alkenes contain at least one double bond between carbon atoms (C=C) and have a general formula of CnH2n. The double bond introduces reactivity and allows alkenes to undergo various reactions, such as addition reactions.
Alkynes: Alkynes possess at least one triple bond between carbon atoms (C≡C) and have a general formula of CnH2n-2. The triple bond makes alkynes even more reactive than alkenes and enables them to participate in a variety of chemical transformations.
ii. Aromatic Hydrocarbons: Cyclic and Delocalized Structure
Aromatic hydrocarbons are characterized by their cyclic structure, where carbon atoms are arranged in a closed ring. They also exhibit delocalization, a phenomenon where electrons are spread out over the ring, giving them unique stability and properties. Common examples of aromatic hydrocarbons include benzene (C6H6), toluene (C7H8), and naphthalene (C10H8).
iii. Significance of Hydrocarbon Classification
Classifying hydrocarbons as aliphatic and aromatic is essential for several reasons:
Understanding Properties: The classification of hydrocarbons helps in understanding their physical and chemical properties. Aliphatic hydrocarbons generally have lower boiling points and are less reactive than aromatic hydrocarbons.
Predicting Reactivity: Knowing whether a hydrocarbon is aliphatic or aromatic allows chemists to predict its reactivity in various chemical reactions.
Systematic Nomenclature: Classification facilitates the systematic naming of hydrocarbons using the IUPAC nomenclature system.
The classification of hydrocarbons into aliphatic and aromatic provides a fundamental framework for understanding the structure, properties, and reactivity of these essential organic compounds. By recognizing the open-chain or branched-chain nature of aliphatic hydrocarbons and the cyclic and delocalized structure of aromatic hydrocarbons, chemists can navigate the vast world of hydrocarbons and unlock their secrets.